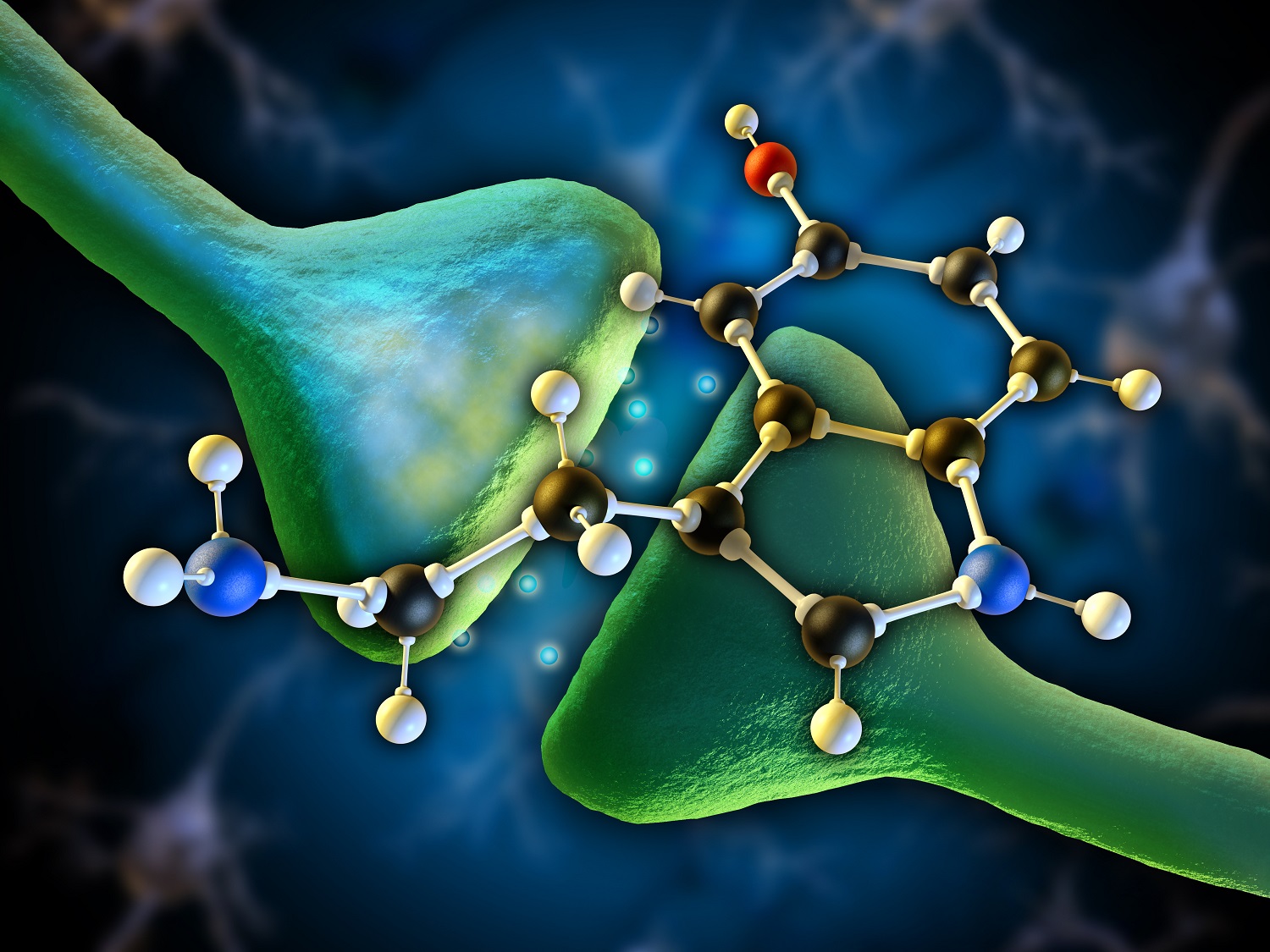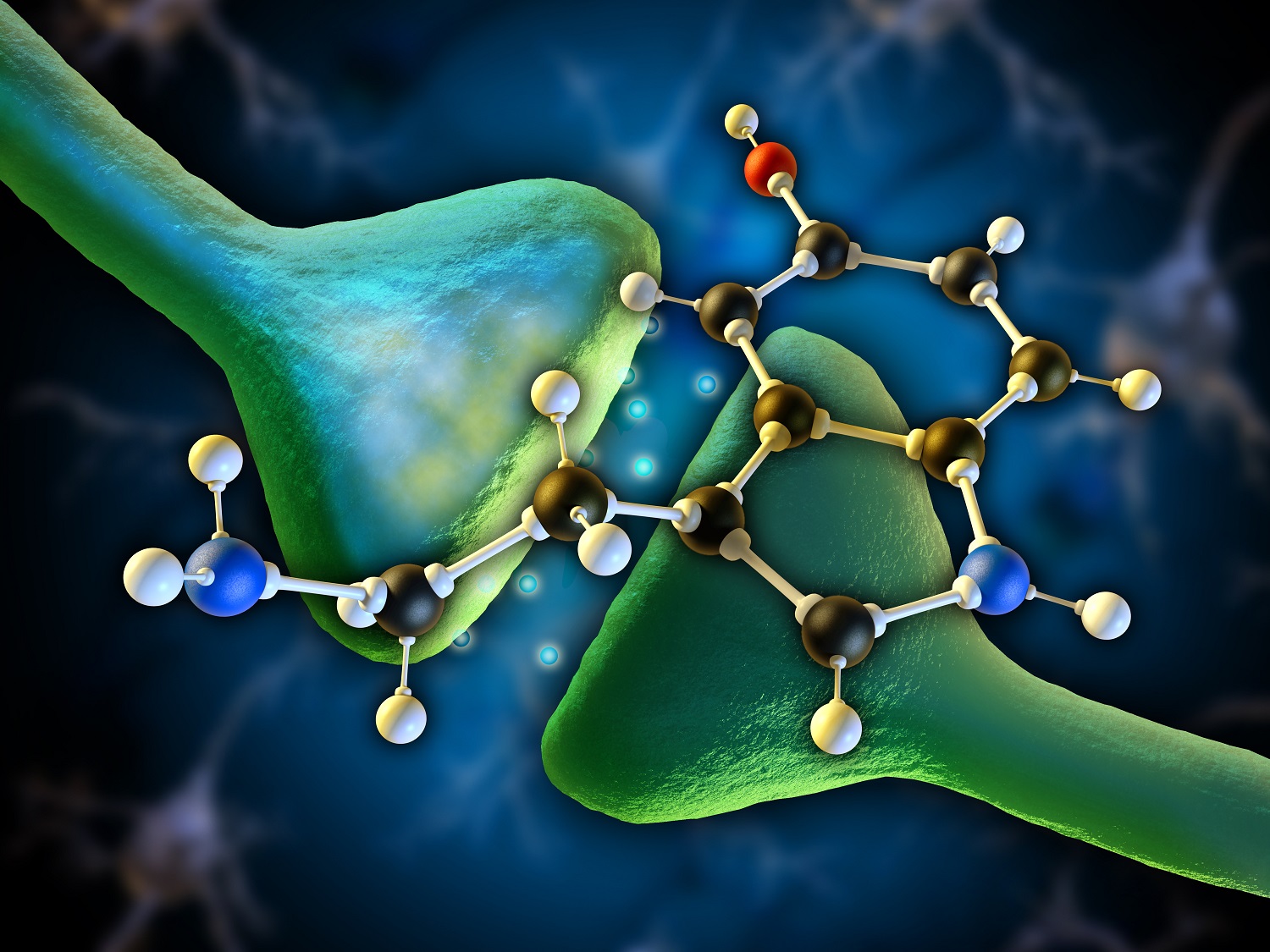
When the Greek travel writer Pausanias visited Delphi, he noted that the axiom “know thyself” [γνῶθι σεαυτόν] was inscribed in the pronaos of the main temple consecrated to Apollo. Various interpretations have been offered throughout the years–everything from dire warnings against the hubris of ego inflation to the pitfalls inherent in pandering to the opinion of the multitude. Beyond that a more conscientious evaluation of possible implications afforded by this ancient maxim will throw light upon another unconscious but equally important aspect of human functioning, our neurobiological constitution. It has been demonstrated that the aminergic system plays a role in mediating conscious states like rational analysis, focal attention, judgment, and semantic language while at the same time mediating brainstem functions via the sympathetic branch of the Central Nervous System (CNS) (Hobson, 1994). In shifting input strength from information encoded in the brain as memory, or internal processing, to information being received through the sensory faculties, or external stimulation, the amines can correct projection and misperceptions through attentional direction (Hobson, 1994).
Prior research has unearthed correlations between the aminergic system, specifically the monoamine serotonin, and affective processing (Cools, Roberts & Robbins, 2008). In particular, psychopharmacological investigations have linked reductions of serotonin in clinical populations to anxiety (Deakin, 1998), stress (Vollmayr, Keck, Henn & Schloss, 2000), negative mood (Van der Does, 2001), and depression (Deakin, Pennell, Upadhyaya & Lofthouse, 1990); Owens & Nemeroff, 1994). Over the last ten years, investigations have become much more comprehensive, examining the beneficial effects of serotonin reuptake inhibitors and norepinephrine reuptake inhibitors like citalopram and reboxetine (both are antidepressants) on the emotional affects and behaviors of perfectly healthy, socially functional volunteers (Tse & Bond, 2001; Harmer, Hill, Taylor, Cowen, & Goodwin, 2003; Harmer, Shelley, Cowen & Goodwin, 2004; Brummett, Boyle, Kuhn, Siegler, & Williams, 2009Crockett, Clark, Hauser & Robbins, 2010). These studies all vouch for a plausible relationship between the aminergic system, sanguine mood, and prosocial behaviors. Understanding when the amines are operating at optimum efficiency and output and how these brain-mind states might be maintained bestows us with newfound latitudes and acumen from whence we can act proactively to preserve our positive moods. Consequently, the likelihood of engaging in prosocial communion and constructive, productive, and mutually satisfying social linkages increases exponentially. Contrary to what some may believe, we are directly responsible for our psychological health along with the empathic resonance and quality of our interpersonal connections in the social synapse. Echoing the subtle mental prompts offered by our ancient ancestors, it is vital that we “know thyself”.
Prosocial behaviors comprise intentional acts that somehow benefit others. These are altruistic in nature and are explicitly defined as being beneficial to others–including society as a whole–but not to self. Comforting, helping, sharing, reassuring, defending, and expressing concern for another are all nuances of prosocial orientation (Schroeder, Penner, Dovidio, & Piliavin, 1995). On the whole, social psychologists have discovered that positive and cheerful moods facilitate prosocial behaviors (Cunningham, Steinberg & Grev, 1980; Cunningham, Steinberg & Barbee, 1990; Eisenberg, 1991). The maintenance of a cheerful mood, or the perception that one’s good mood will be enhanced or preserved, seems to be an imperative factor in determining whether an individual will engage in prosocial behavior or not. It has come to light that when individuals have arrived at sanguine states through serendipitous strokes of luck (i.e. the reception of money, gifts, and personal instruction) they will almost always pick up books and papers accidentally dropped onto the ground, offer their time and money, conduct extensive searches for lost contact lenses, donate blood, and give intellectual guidance to penurious students (Salovey, Mayer & Rosenhan, 1991). An individual who remains pleased and contented is far more likely to arrive at a positive appraisal for a situation in which another requires assistance than one who is not (Clark & Waddell, 1983). Moreover, those subsisting in positive moods are more likely to experience attraction and form positive characterological impressions and evaluations of others (Isen, Shalker, Clark & Karp, 1978). Of course one’s readiness to provide assistance is also modulated by other competing factors like personality, the particular circumstances configured by the immediate environment, and autobiographical history. To this we could safely add aminergic activation.
Understanding which cerebral state is conducive to positive mood requires an adequate understanding of the human biorhythm, a phenomenon to which we will now turn. The first thing we must become cognizant of is that the band of conscious states we might experience–everything from light relaxation and alertness to daydreaming, dreaming, and psychosis–are buttressed by corresponding neurochemical conditions in the brain, with this mind-brain correlation easily charted via sophisticated neuroimaging technology like Functional Magnetic Resonance Imaging (fMRI), Positron Emission Tomography (PET), and Electroencephalography (EEG) (Aine, 1994). For each mental state, then, there is a corresponding neurochemical correlate. Even the slightest variation at the chemical level has far-reaching consequences for the overall form (the higher cognitive faculties, including perception, attention, memory, and orientation as well as the limbic faculties, namely emotion) and content of consciousness (Hobson, 1994). Depending on the manner in which global firing patterns of cortical and subcortical neurons are altered, a chemical shift may translate to any of the following mental states: deep relaxation, a transient psychotic episode, a vivid hallucination, or a complete dissipation of consciousness such as that experienced during coma and non-REM sleep.
Our cognitive and emotional lives swing like giant pendulums between two polarities which cannot be transcended; on one end we have gross insensitivity to the external world, a phenomenon frequently labelled unconsciousness, and on the other, a self-awareness orientated to time, place, and other persons and characterized by an unwavering ability to scrupulously direct attention towards whatever we choose. Both higher brain functions like thoughts, insight, rational analysis, judgment, and semantic language and lower brain functions like somatic sensations and movement generated through the muscles are made possible by two neurotransmitter systems known as the catecholamines–dopamine, norepinephrine, and epinephrine–and the cholines, explicitly acetylcholine (Hobson, 1994). Shared but differentiated characteristics enable one system to expend energy by speeding up the metabolic processes responsible for neural network activation and the other to conserve energy by slowing them (Gazzaniga, Ivry, & Mangun, 2014). Together they mediate the sleep-wake cycle, a biological expression of the yin-yang cycle.
Collectively the catecholamines form the aminergic system; they are excitatory, ergotropic in nature, and intimately linked with the internal processing of sensory representations gathered from the external environment (Gazzaniga et al., 2014). Their central headquarters can be found in the primitive brain, the brainstem. The neural circuitry linking to form the serotonin system, for instance, is couched within an important part of the brainstem called the pons but its reach is widescale, projecting up into the cortex, the mediator of higher cognitive functions, and down into the spinal cord. Its significant scope implies a global level of control over psychophysiological functions (Hobson, 1994). Brainstem cells that utilize norepinephrine are fundamental to focal attention (Bunsey & Strupp, 1995; Clark, Geffen & Geffen, 1989). When the amines are firing on all cylinders we find ourselves receptive to external representations carried to the brain via our sensory modalities; we’re able to maintain concentration for protracted periods without reprieve; and we’re mentally agile, able to hold a thought or image in our minds, pay attention to detail, perform mathematical computations, deploy self-reflection and analysis, and solve complex problems at accelerated speeds (Gazzaniga et al., 2014). More importantly so, we are more than likely to be in a cheerful and optimistic mood (Brummett, Boyle, Kuhn, Siegler, & Williams, 2009; Flory, Manuck, Matthews & Muldoon, 2004). A high ratio of amines to cholines denotes the imposition of foreground processing or external stimulation on information already encrypted in the cerebral synapses as memory (Clark et el., 1989). During this time it is possible for intricate and overlearned behaviors associated with the latter to be amended via focal attention.
On the other hand what happens when aminergic activity in the brain starts to dip? There is depreciative shift in the form and content of consciousness; our ability to fixate upon internal or external targets for indefinite periods wavers, we grow lethargic, and our capacity for selective attention becomes chaotic and undisciplined. Focusing on a moderately difficult task may become nearly impossible. When electrical activation and input strength had favored serotonin and norepinephrine we were steadily anchored to reference points bequeathed by the consensus environment, but now an increase in cholinergic tension has shifted input to an internal perceptual mode, initiating an unbounded regress to the internal world of imagery from where microhallucinations and misperceptions are bound to arise (Clark et al., 1989). For the brain, a drop in norepinephrine levels and a rise in cholinergic tension during diurnal hours have been correlated with an increase in the internally-generated mental images of daydreaming; with projection; and with increased anxiety (Meissner, 2008). This is when we’re most likely to ruminate, confabulate, and ponder plans and existing reference frames. Remaining adrift in these states gives others the impression that we’re trapped within our artificial worlds, at least until a thunderous clap, thump, or scream snaps us from our provisional impairment. A further loss in aminergic thrust brings us to states of very deep relaxation and hypnosis, close to the equivocal fringes of hypnagogic slumber. When the amines in the pons come perilously close to complete inertia, consciousness dissipates and we involuntarily enter into the abode of non-REM sleep (Hobson, 2011).
Slipping into non-REM sleep reflects an electrochemical deadlock between the aminergic and the cholinergic systems (Hobson, 1994). Neurons belonging to the second family are inhibitory, trophotrophic in nature, and directly responsible for REM-mode sleep (Hobson, 2011). Dreams themselves cannot manifest in consciousness unless the cholinergic system sets off field potentials by washing over a deep brainstem region called the pons, a visual relay station of the subcortical thalamus named the lateral geniculate nucleus, and the occipital lobe of the visual cortex (Hobson, 2011); when cholinergic excitation and a reciprocal inhibition of the aminergic neurons in the pons becomes the predominant brain state, we become like colourful puppets on interlinking chords and threads, dancing as it were to the inner orchestra of our own unconscious content. A temporary switch from prefrontal to brainstem control, from cortical to subcortical activation, is fully responsible for the arbitrary cognitive deficits in our internally generated perceptions.
Condensing clinical observations into a succinct yet accurate description we might say that episodes of REM-bound sleep are characterized by several consistent features–severe disorientation and hyperassociation; an absorption and distractibility in a dreamscape likely to violate Aristotelian homogeneity; poor memory recall and confabulation; deficits in rational analysis and insight; implied language; visual-motor perceptions with obvious cinematic and surrealistic aspects couched in vivid hallucinations and delusions; and an unhindered expression of mixed emotions like fear, anger, joy, sadness, remorse, and shame (Hobson, 1994). The self or ‘I’ is back, albeit with severely limited degrees of freedom. We do not have creative control over the visual-motor narratives perceived and we cannot enforce or direct our own will. For the most part we’ll just blend into an ambient background of subjective fields, flitting about here, there, and everywhere without as much as a hunch for where we’re going or what we’re doing. Our minds seem to be made up, manoeuvred, and managed for us without our choosing. In retrospect we’re exactly like Plato’s prisoners who were forced to sit and observe an unintelligible shadow-play projected onto a wall before them.
After about four or five nocturnal cycles of intermittent REM and non-REM periods, a sudden but powerful recourse to norepinephrine initiated by the brainstem reinstates top-down processing, snapping the individual wide awake. We’re now free to pick up where we left off before falling asleep, thanks to the successful rebooting of the aminergic system and the subsequent activation of the neocortex (Jouvet, 1972). Complete rejuvenation of the amines and immediate suppression of acetylcholine means seizure-like REM-mode hallucinations have been arrested and the reactivated prefrontal cortex can once again synthesize external and internal input into an integrated perception that reproduces the world but doesn’t go so far as to create entirely artificial worlds. At this moment in time we have repossession of our ‘I’ function, the conscious awareness which mediates our lives by the light of day. We’re also at our most alert and energetic, implying that we’re also likely to be experiencing affirmative moods. The central hypothesis here is that of the amines are soaring high, so too will temperament and mood be skewed towards the positive, the optimistic, and the cheerful.
Let’s briefly consider some clinical discoveries that support a connection between the monoamines and mood. The neurotransmitter serotonin, for instance, is synthesized by neurons in the raphe nucleus of the brainstem which project into the cortical networks and control cognition, emotion, and pain sensitivity. A reduction of mRNA levels in the raphe nucleus of serotonergic neurons has been linked to anxiety (Deakin, 1998), stress (Vollmayr, Keck, Henn & Schloss, 2000), negative mood (Van der Does, 2001), and depression (Deakin, Pennell, Upadhyaya & Lofthouse, 1990); Owens & Nemeroff, 1994). Falling within the theoretical scope of the psychiatric disorders, bulimia, chronic impulsivity, aggression, suicide, and obsessive-compulsive disorders have all been positively associated with disruptions in serotonergic signalling (Baumgarten and Grozdanovic, 1995; Hen, 1996; Berman, Tracy & Coccaro, 1997).; Mann, 1998). More significantly, the treatment for these conditions will often involve pharmacological agents which act to increase concentrations of serotonin in the brain by inhibiting reuptake at the synaptic junctions. It has also been established that antidepressants cannot function in the human adult unless serotonin has stimulated hippocampal neurogenesis and synaptic plasticity first (Brezun & Daszuta, 1999, 2000; Santarelli et al, 2003).
Psychopharmacological interventions with perfectly healthy individuals have successfully examined antidepressant action on psychological mechanisms unencumbered by significant mood improvement and symptom remission, shedding light upon the instrumental role catecholamines play in the expression of positive affect. One has examined the relation between reactivity and levels of norepinephrine in the blood and positive affect during periods of emotional recall (Brummett, Boyle, Kuhn, Siegler, & Williams, 2009). The experimental sample included 328 subjects free from major medical conditions, psychiatric disorders, and pregnancies, who were critically assessed on qualitative measures for positive affect and quantitative ones for age, race, sex, smoking status, income level, and BMI. Dispositional tendencies to negative and positive affect were quantified using the Profile of Mood States (POMS) (McNair & Loir 1964); the Visual Analog Scale (VAS); and the NEO-PI-R (Costa & McCrae, 1992). The emotional recall protocol involved pondering a past experience that had and still evoked anger, and subsequently visualizing it. Inserted prior to the recollection exercise, an in-dwelling catheter collected blood samples following the 10-minute baseline period, the anger recall task, and at the termination of the protocol. The blood was spun in a refrigerated centrifuge, plasma extracted, and the norepinephrine levels therein calculated using high-pressure liquid chromatography (HPCC) followed by electrochemical detection. The reactivity protocol for each of the three measurement periods revealed that higher levels of positive affect corresponded with decreases in plasma catecholamines. In hindsight, the data suggest bidirectional causation between positive mood and conservation of the monoamines.
Another experimental trial incorporating double-blind interventions examined the effects of the selective serotonin reuptake inhibitor (SSRI) citalopram, the selective norepinephrine reuptake inhibitor reboxetine (SNRI), and a placebo in forty-two volunteers over a seven-day period (Harmer, Shelley, Cowen & Goodwin, 2004). After receiving one of placebo, citalopram (40mg/day), or reboxetine (4mg/day), the day-by-day variations in the volunteers’ moods was measured with the assistance of the Befindlickkeits scale of mood and energy. What is more, participants were subjected to questionnaires including the State-Trait Anxiety Inventory, the Beck Depression Inventory, the Positive and Negative Affect Schedule, and the Social Adaptation Self-Evaluation Scale before and after the week-long intervention. On the final day a family of cognitive tasks designed to ascertain levels of depression and anxiety were deployed: the emotion-potentiated startle response; facial expression perception; emotional categorization; and emotional memory.
It appears that individuals in both experimental groups experienced significant decreases in angry (citalopram: t = 2.0, df = 26, p = 0.055; reboxetine: t = 2.1, df = 26, p = 0.04) and fearful (citalopram: t = 2.0, df = 26, p = 0.055; reboxetine: t = 2.1, df = 26, p = 0.04) facial expressions in comparison to the placebo, indicating a switch to positive bias in the emotional appraisal of facial expressions. Those who were in the citalopram group also experienced significant reductions in facial recognition expressions of disgust (t = 3.5, df = 26, p = 0.002) and surprise (t = 3.0, df = 26, p = 0.006), as well as the complete extirpation of the emotion-potentiated startle response to negative affective images (F = 1.7, df = 2, 30, p > 0.20). Both antidepressants increased the relative recall of positive to negative emotional material, whereas no statistically significant change was seen with the placebo. If an artificial efflux of serotonin and norepinephrine inaugurated by antidepressants can enforce positive alterations to perceptual categorization and memory in the brains of nonclinical healthy volunteers, then who is to say that it should not also influence subjective mood. When it comes to the processing of social information, focal attention accompanied by positive bias usually precedes and facilitates the propagation of positive mood.
Another similar study (Harmer, Hill, Taylor, Cowen, & Goodwin, 2003) with a randomized, double-blind, and between-groups design subjected twenty-four healthy volunteers to a single dose of the antidepressant reboxetine–the same norepinephrine receptor inhibitor that was utilized in the aforementioned study–for the sake of seeing what the effects would be on their emotional processing. Two hours after receiving either 4mg of reboxetine or a placebo, participants were required to undertake a series of psychological tests involving the recognition of facial expressions, emotional memory, and emotional categorization. They were also required to take the Rapid Visual Information Processing Task. The results revealed that those in the experimental group experienced greater differences in their reactions to positive and negative descriptors, remembered a greater number of positive descriptors, and were more likely to recognize positive or cheerful facial expressions (F = 8.1, df = 4.88, p < 0.001) than those in the placebo group. Quintessentially the active chemical efflux initiated by the antidepressant reboxetine translated to a subtle shift in internal perception so that positively valenced emotional information could be expedited without any discernible change to cognitive performance. Resounding the finds and implications of the previous experiment, pharmacological modulation of the monoamines may skew processing biases (either external/foreground or internal/background) towards the positive, however the full-fledged development of improved subjective mood may require something more, perhaps a subsequent empathic involvement in interpersonal and intergroup transactions.
Tse and Bond (2001) took the investigation a step further into a novel direction and examined how the acute manipulation of the neurotransmitters serotonin and noradrenalin might impact the social behaviors of nonclinical, healthy volunteers. A total of sixty participants were gathered and assigned to one of three treatment groups: a reboxetine (4 mg) group, a citalopram group (10 mg), and a placebo. Roughly one and a half hours after ingestion, volunteers were ushered into a room and requested to commune with a nonsociable and somewhat awkward sex-matched confederate (volunteer) before engaging in a mixed-motive computer game with him or her. The latter was supposed to determine levels of communication and cooperative behavior. Subjects were also provided with several questionnaires intending to quantify mood, affect, and anxiety which they completed before the treatment, immediately before the dyadic social interaction, and afterwards. It came to light that individuals on reboxetine demonstrated more cooperative behavior in the mixed-motive game than either those in the citalopram group or the placebo (X2 = 5.95, p < 0.05). In addition there was a statistically significant reduction in hand fidgeting for the former but not the latter. A post-trial speech analysis revealed less reduction in the energy fluctuations of participants in the citalopram group for a reading task. Evident from the consequent behaviors is that the increases in endogenous norepinephrine generated by an acute dose of reboxetine reduced anxiety; it increased alertness and cooperative disposition; and it blighted negatively charged non-verbal signals likely to be perceived in an individual who has just tried to interact with a socially withdrawn other. The amines may indeed regulate and promote prosocial behaviors, albeit indirectly.
More recently, significant increases in the monoamine serotonin were connected to the augmentation of aversive reactions to the fantasy or reality of inflicting grievous harm upon other humans, reflecting the former’s involvement in the sharpening of moral judgment and prosocial behaviors (Crockett, Clark, Hauser & Robbins, 2010). The double-blind fully counterbalanced design involved three trials spread over three weeks. Twenty-four healthy volunteers including both men and women were selected and then divided into three groups. Single oral doses of citalopram (30mg) and atomoxetine (60mg) were given to the experimental and pharmacological control groups, respectively, while the control group received sugar pills disguised to look like antidepressants. The Positive and Negative Affect Scale and visual analog scales were used to assess mood and subjective characteristics and attitudes. Some one and a half hours after ingestion, the volunteers were subjected to a cognitive testing battery that included cognitive function tasks, playing the ultimatum game, and moral judgment. The experimenters induced scenarios that were nonmoral, emotionally salient and personal, or less emotionally salient and impersonal via computer interface, recorded responses pertaining to the assessment of harmful actions, and subsequently compared them.
There were nonsignificant variations in moral judgments made by the experimental group, the pharmacological control group, and the placebo for neutral and impersonal scenarios. Nonetheless, a harm-avoidant bias colored the perceptions of individuals on citalopram when the scenario was both personal and emotionally salient. Relative to the mean choices made by individuals in the atomoxetine and control groups, exposure to citalopram meant that you were much less likely to reject prejudiced and unjust monetary offers made by other players in the ultimatum game. Because the rejection of unfair offers was purposely coupled with imposed financial deficits for the playing partner, it might be inferred that those with increased endogenous serotonin sacrificed the just and the morally right decision to avoid imposing unintentional detriments upon others. As predicted by the researchers’ hypothesis, stronger cognitive and behavioral effects were salient across the full set of tasks for individuals scoring highly in trait empathy relative to those who scored lower on the same measure. Collectively the findings support the contention that augmenting serotonin in a healthy, nonclinical population promotes prosocial behavior at the individual level by increasing harm aversion.
Owing to the fact that clinical neuroscience is still in its infancy the exact cortical and subcortical pathways facilitating these existing neuropsychosocial phenomena are yet to be identified. On the other hand clinical evidence is firmly in support of a causal relationship between the monoamines serotonin and norepinephrine, mood, and prosocial behavior in human beings. Whether we wish to admit it or not, the ebbs and flows of cerebral neurotransmitters modulate the perception, emotion, orientation, and memory within each integrated mental state which then determine, to some degree or another, whether we will act in an antisocial or prosocial manner. The overarching implication here, at least from a health science perspective, is that by entering trophotropic mode through sleep and diurnal napping on a regular basis we rejuvenate the aminergic neurons, meaning we awake feeling vibrant and attentive. If we’re feeling refreshed and alert, chances are that we’re also in an elevated and optimistic mood, increasing the likelihood that our own interpersonal dealings will be characterized by mindful awareness, empathic attunement, and integrative communication. Offering an education on the internal biorhythms responsible for homeostatic balance; on optimum psychosomatic functionality; and on the underlying connections between three distinct ontologies (the neural, psychological, and social) empowers each and every one of us to act volitionally and proactively in the conservation of our own health.
Bibliography
Aine, C. J. (1994). A conceptual overview and critique of functional neuroimaging techniques in humans: I. MRI/FMRI and PET. Critical reviews in neurobiology, 9(2-3), 229-309.
Baumgarten, H. G., & Grozdanovic, Z. (1995). Psychopharmacology of central serotonergic systems. Pharmacopsychiatry.
Berman M. E., Tracy, J.I., & Coccaro E.F. (1997). The serotonin hypothesis of aggression revisited. Clinical Psychology Review, 17: 651–665.
Brezun, J. M., & Daszuta, A. (1999). Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience, 89(4), 999-1002.
Brezun, J. M., & Daszuta, A. (2000). Serotonin may stimulate granule cell proliferation in the adult hippocampus, as observed in rats grafted with foetal raphe neurons. European Journal of Neuroscience, 12(1), 391-396.
Brummett, B. H., Boyle, S. H., Kuhn, C. M., Siegler, I. C., & Williams, R. B. (2009). Positive affect is associated with cardiovascular reactivity, norepinephrine level, and morning rise in salivary cortisol. Psychophysiology, 46(4), 862-869.
Bunsey, M. D., & Strupp, B. J. (1995). Specific effects of idazoxan in a distraction task: evidence that endogenous norepinephrine plays a role in selective attention in rats. Behavioral neuroscience, 109(5), 903.
Clark, C. R., Geffen, G. M., & Geffen, L. B. (1989). Catecholamines and the covert orientation of attention in humans. Neuropsychologia, 27(2), 131-139.
Clark, M. S., & Waddell, B. A. (1983). Effects of moods on thoughts about helping, attraction and information acquisition. Social Psychology Quarterly, 31-35.
Cools, R., Roberts, A. C., & Robbins, T. W. (2008). Serotoninergic regulation of emotional and behavioural control processes. Trends in cognitive sciences, 12(1), 31-40.
Crockett, M. J., Clark, L., Hauser, M. D., & Robbins, T. W. (2010). Serotonin selectively influences moral judgment and behavior through effects on harm aversion. Proceedings of the National Academy of Sciences, 107(40), 17433-17438.
Cunningham, M. R., Shaffer, D. R., Barbee, A. P., Wolff, P. L., & Kelley, D. J. (1990). Separate processes in the relation of elation and depression to helping: Social versus personal concerns. Journal of Experimental Social Psychology, 26(1), 13-33.
Cunningham, M. R., Steinberg, J., & Grev, R. (1980). Wanting to and having to help: Separate motivations for positive mood and guilt-induced helping. Journal of Personality and Social Psychology, 38(2), 181.
Deakin, J. F. (1998). The role of serotonin in panic, anxiety and depression. International clinical psychopharmacology.
Deakin, J. F. W., Pennell, I., Upadhyaya, A. J., & Lofthouse, R. (1990). A neuroendocrine study of 5HT function in depression: evidence for biological mechanisms of endogenous and psychosocial causation. Psychopharmacology, 101(1), 85-92.
der Does, V., & Willem, A. J. (2001). The effects of tryptophan depletion on mood and psychiatric symptoms. Journal of affective disorders, 64(2), 107-119.
Duman, R. S. (2004). Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular medicine, 5(1), 11-25.
Eisenberg, N. (1991). Meta-analytic contributions to the literature on prosocial behavior. Personality and Social Psychology Bulletin, 17(3), 273-282.
Fiske, S. T. (2009). Social beings: Core motives in social psychology. John Wiley & Sons.
Flory, J. D., Manuck, S. B., Matthews, K. A., & Muldoon, M. F. (2004). Serotonergic function in the central nervous system is associated with daily ratings of positive mood. Psychiatry Research, 129(1), 11-19.
Gazzaniga, M. S., Ivry, R. B., & Mangun, G. R. (2014). Cognitive neuroscience: The biology of the mind. Horton, New York.
Harmer, C. J., de Bodinat, C., Dawson, G. R., Dourish, C. T., Waldenmaier, L., Adams, S., & Goodwin, G. M. (2011). Agomelatine facilitates positive versus negative affective processing in healthy volunteer models. Journal of Psychopharmacology, 25(9), 1159-1167.
Harmer, C. J., Hill, S. A., Taylor, M. J., Cowen, P. J., & Goodwin, G. M. (2003). Toward a neuropsychological theory of antidepressant drug action: increase in positive emotional bias after potentiation of norepinephrine activity. American Journal of Psychiatry, 160(5), 990-992.
Harmer, C. J., Shelley, N. C., Cowen, P. J., & Goodwin, G. M. (2004). Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. American Journal of Psychiatry, 161(7), 1256-1263.
Hen, R. (1996). Mean genes. Neuron. 16: 17–21.
Hobson, J. A. (2005). Dreaming: a very short introduction. Oxford University Press.
Hobson, J. A. (1994). The chemistry of conscious states. Back Bay Books.
Isen, A. M., & Levin, P. F. (1972). Effect of feeling good on helping: cookies and kindness. Journal of personality and social psychology, 21(3), 384.
Isen, A. M., Shalker, T. E., Clark, M., & Karp, L. (1978). Affect, accessibility of material in memory, and behavior: A cognitive loop?. Journal of personality and social psychology, 36(1), 1.
Jouvet, M. (1972). The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. In Neurophysiology and Neurochemistry of Sleep and Wakefulness (pp. 166-307). Springer Berlin Heidelberg.
Kelley, S. W., & Hoffman, K. D. (1997). An investigation of positive affect, prosocial behaviors and service quality. Journal of Retailing, 73(3), 407-427.
Lyubomirsky, S., King, L., & Diener, E. (2005). The benefits of frequent positive affect: does happiness lead to success?. Psychological bulletin, 131(6), 803.
Mann, J. J. (1998). The role of in vivo neurotransmitter system imaging studies in understanding major depression. Biological psychiatry, 44(11), 1077-1078.
Meissner, W. W. (2008). Mind-brain and consciousness in psychoanalysis. Bulletin of the Menninger Clinic, 72(4), 283-312.
Owens, M. J., & Nemeroff, C. B. (1994). Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clinical chemistry, 40(2), 288-295.
Salovey, P., Mayer, J. D., & Rosenhan, D. L. (1991). Mood and helping: Mood as a motivator of helping and helping as a regulator of mood.
Santarelli, L., Saxe, M., Gross, C., Surget, A., Battaglia, F., Dulawa, S., & Hen, R. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science, 301(5634), 805-809.
Vollmayr, B., Keck, S., Henn, F. A., & Schloss, P. (2000). Acute stress decreases serotonin transporter mRNA in the raphe pontis but not in other raphe nuclei of the rat. Neuroscience letters, 290(2), 109-112.
Wai, S. T., & Bond, A. J. (2002). Difference in serotonergic and noradrenergic regulation of human social behaviours. Psychopharmacology, 159(2), 216-221.